Exactech Product Liability Cases
Exactech Product Liability Cases
Arthroplasty has become one of the most performed and successful surgeries in recent times. The frequency with which this procedure is performed has steadily increased over time, as has the variety of manufactured prosthetic products used. These products serve a specific function, designed with the intention to work synergistically to replace the intended joint (hip, knee, ankle) successfully and for a long duration, complication-free. Several issues can arise from faulty products implanted in patients which may manifest on the scale of months to years post-surgery. Highly cross-linked polyethylene was developed to address the problem of wear and osteolysis associated with metal-on-conventional ultra-high molecular weight polyethylene (UHMWPE) bearing surfaces.
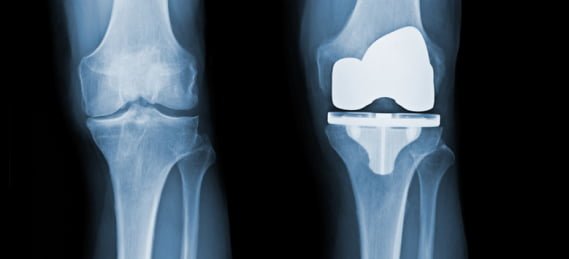
Total Knee Replacement (TKR) consists of resection of the diseased articular surfaces of the knee, followed by resurfacing with metal and polyethylene prosthetic components. Generally, TKR is performed for the destruction of joint cartilage either from osteoarthritis (degeneration of joint cartilage and the underlying bone), rheumatoid arthritis/inflammatory arthritis (inflammation in the joints with painful deformity), post-traumatic degenerative joint disease, or osteonecrosis/joint collapse (death of bone tissue due to a lack of blood supply) with cartilage destruction.
A standard total knee replacement has four parts: 1. The femoral component (this is the metal piece that attaches to the thigh bone, also known as the “femur”); 2. The tibial tray (this is the metal piece that fits into the shin bone, also known as “tibia”); 3. The patellar component (this is the piece of plastic that fits into the kneecap, also known as the patella); 4. The tibial polyethylene (plastic) insert (this is the plastic that fits between the femoral component and tibial component and acts as the new cushion or cartilage for the replaced knee joint).
Findings
Exactech alleges that the premature degradation of the polyethylene components in its knee replacement systems is not the proximate cause to such issues. Instead, it is the inadequate packaging, non-conforming packaging lacking a secondary barrier that confers additional resistance. Exactech believes that this packaging may enable increased oxygen penetration to the system and that over time “oxidation can severely degrade the mechanical properties of conventional UHMWPE, which, in conjunction with other surgical factors, can lead to both accelerated wear debris production and bone loss, and/or component fatigue cracking/fracture, all leading to corrective revision surgery.”
Regardless, Exactech Ultra-High Molecular Weight Polyethylene (UHMWPE) Knee Polyethylene components have shown premature wear and potential concerns, reaching enough reported cases and conditions to grant a recall and investigation by the FDA.
Litigation Status
Investigations by the FDA remain underway and extensive litigation can be expected to commence shortly. While there have been individual suits ongoing since 2017, no class action lawsuit has yet to be organized. The proximate cause will certainly be the main issue in these cases, making this product liability issue one to watch.
Expert Witness Opportunities
Experts in biomedical engineering are the key specialists for this type of litigation. Biomedical engineers would be able to opine on how the implants are designed. They would also be able to offer the extent to which the potential failure points were known (or unknown) and what kind of harm they could bring patients in the long term.
Get Help now!
If you or a loved one have been injured, you may be eligible for a lawsuit. A lawsuit can help recover damages for medical costs, pain and suffering, and more.
Contact us today for a free, no-obligation legal review.